
_________________________________________________________________
_____________________________
___________________________________________________________________________________________________
Page 1
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to
use Plan B One-Step safely and effectively. See full
prescribing information for Plan B One-Step.
Plan B One-Step (levonorgestrel) tablet, 1.5 mg, for oral use
Initial U.S. Approval: 1982
----------INDICATIONS AND USAGE--------------
Plan B One-Step is a progestin-only emergency contraceptive indicated for
prevention of pregnancy following unprotected intercourse or a known or
suspected contraceptive failure. Plan B One-Step is available only by
prescription for women younger than age 17 years, and available over the
counter for women 17 years and older. Plan B One-Step is not intended for
routine use as a contraceptive. (1)
-------DOSAGE AND ADMINISTRATION--------
One tablet taken orally as soon as possible within 72 hours after unprotected
intercourse. Efficacy is better if the tablet is taken as soon as possible after
unprotected intercourse. (2)
-------DOSAGE FORMS AND STRENGTHS-----
1.5 mg tablet (3)
-----------CONTRAINDICATIONS-----------------
Known or suspected pregnancy (4)
-------WARNINGS AND PRECAUTIONS---------
• Ectopic pregnancy: Women who become pregnant or
complain of lower abdominal pain after taking Plan B One-
Step should be evaluated for ectopic pregnancy. (5.1)
• Plan B One-Step is not effective in terminating an existing
pregnancy. (5.2)
• Effect on menses: Plan B One-Step may alter the next
expected menses. If menses is delayed beyond 1 week,
pregnancy should be considered. (5.3)
• STI/HIV: Plan B One-Step does not protect against
STI/HIV. (5.4)
----------------ADVERSE REACTIONS--------------
The most common adverse reactions (≥10%) in clinical trials
included heavier menstrual bleeding (31%), nausea (14%), lower
abdominal pain (13%), fatigue (13%), headache (10%), and
dizziness (10%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact
Barr Laboratories at 1-800-330-1271 or FDA at 1-800-FDA-
1088 or www.fda.gov/medwatch.
----------------DRUG INTERACTIONS--------------
Drugs or herbal products that induce certain enzymes, such as
CYP3A4, may decrease the effectiveness of progestin-only pills. (7)
-----------USE IN SPECIFIC POPULATIONS-----
• Nursing Mothers: Small amounts of progestin pass into the breast milk
of nursing women taking progestin-only pills for long-term
contraception, resulting in detectable steroid levels in infant plasma.
(8.3)
• Plan B One-Step is not intended for use in premenarcheal (8.4) or
postmenopausal females (8.5).
See 17 for PATIENT COUNSELING INFORMATION.
Revised 7/2009
FULL PRESCRIBING INFORMATION:
CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Ectopic Pregnancy
5.2 Existing Pregnancy
5.3 Effect on Menses
5.4 STI/HIV
5.5 Physical Examination and Follow-up
5.6 Fertility Following Discontinuation
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Race
8.7 Hepatic Impairment
8.8 Renal Impairment
9 DRUG ABUSE AND DEPENDENCE
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment
of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING NFORMATION
17.1 Information for Patients
*Sections or subsections omitted from the full prescribing
information are not listed
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Page 2
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Plan B
®
One-Step is a progestin-only emergency contraceptive indicated for prevention of pregnancy following unprotected intercourse or a known or suspected
contraceptive failure. To obtain optimal efficacy, the tablet should be taken as soon as possible within 72 hours of intercourse.
Plan B One-Step is available only by prescription for women younger than age 17 years, and available over the counter for women 17 years and older.
Plan B One-Step is not indicated for routine use as a contraceptive.
2 DOSAGE AND ADMINISTRATION
Take Plan B One-Step orally as soon as possible within 72 hours after unprotected intercourse or a known or suspected contraceptive failure. Efficacy is better if
the tablet is taken as soon as possible after unprotected intercourse. Plan B One-Step can be used at any time during the menstrual cycle.
If vomiting occurs within two hours of taking the tablet, consideration should be given to repeating the dose.
3 DOSAGE FORMS AND STRENGTHS
The Plan B One-Step tablet is supplied as an almost white, round tablet containing 1.5 mg of levonorgestrel and is marked G00 on one side.
4 CONTRAINDICATIONS
Plan B One-Step is contraindicated for use in the case of known or suspected pregnancy.
5 WARNINGS AND PRECAUTIONS
5.1 Ectopic Pregnancy
Ectopic pregnancies account for approximately 2% of all reported pregnancies. Up to 10% of pregnancies reported in clinical studies of routine use of progestin-
only contraceptives are ectopic.
A history of ectopic pregnancy is not a contraindication to use of this emergency contraceptive method. Healthcare providers, however, should consider the
possibility of an ectopic pregnancy in women who become pregnant or complain of lower abdominal pain after taking Plan B One-Step. A follow-up physical or
pelvic examination is recommended if there is any doubt concerning the general health or pregnancy status of any woman after taking Plan B One-Step.
5.2 Existing Pregnancy
Plan B One-Step is not effective in terminating an existing pregnancy.
5.3 Effects on Menses
Some women may experience spotting a few days after taking Plan B One-Step. Menstrual bleeding patterns are often irregular among women using progestin-
only oral contraceptives and women using levonorgestrel for postcoital and emergency contraception.
If there is a delay in the onset of expected menses beyond 1 week, consider the possibility of pregnancy.
5.4 STI/HIV
Plan B One-Step does not protect against HIV infection (AIDS) or other sexually transmitted infections (STIs).
5.5 Physical Examination and Follow-up
A physical examination is not required prior to prescribing Plan B One-Step. A follow-up physical or pelvic examination is recommended if there is any doubt
concerning the general health or pregnancy status of any woman after taking Plan B One-Step.
5.6 Fertility Following Discontinuation
A rapid return of fertility is likely following treatment with Plan B One-Step for emergency contraception; therefore, routine contraception should be continued or
initiated as soon as possible following use of Plan B One-Step to ensure ongoing prevention of pregnancy.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared
to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Plan B One-Step was studied in a randomized, double-blinded multicenter clinical trial. In this study, all women who had received at least one dose of study
medication were included in the safety analysis: 1,379 women in the Plan B One-Step group, and 1,377 women in the Plan B group (2 doses of 0.75 mg
levonorgestrel taken 12 hours apart). The mean age of women given Plan B One-Step was 27 years. The racial demographic of those enrolled was 54% Chinese,
12% Other Asian or Black, and 34% were Caucasian in each treatment group. 1.6% of women in the Plan B One-Step group and 1.4% in Plan B group were lost
to follow-up.
The most common adverse events (>10%) in the clinical trial for women receiving Plan B One-Step included heavier menstrual bleeding (30.9%), nausea
(13.7%), lower abdominal pain (13.3%), fatigue (13.3%), and headache (10.3%). Table 1 lists those adverse events that were reported in > 4% of Plan B One-
Step users.
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Page 3
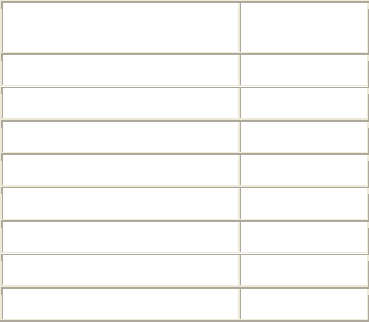
Table 1. Adverse Events in > 4% of Women, by % Frequency
Most Common Adverse Events
(MedDRA)
Plan B One-Step
N = 1359 (%)
Heavier menstrual bleeding 30.9
Nausea 13.7
Lower abdominal pain 13.3
Fatigue 13.3
Headache 10.3
Dizziness 9.6
Breast tenderness 8.2
Delay of menses (> 7 days) 4.5
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Plan B (2 doses of 0.75 mg levonorgestrel taken 12 hours apart). Because these
reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal
relationship to drug exposure.
Gastrointestinal Disorders
Abdominal Pain, Nausea, Vomiting
General Disorders and Administration Site Conditions
Fatigue
Nervous System Disorders
Dizziness, Headache
Reproductive System and Breast Disorders
Dysmenorrhea, Irregular Menstruation, Oligomenorrhea, Pelvic Pain
7 DRUG INTERACTIONS
Drugs or herbal products that induce enzymes, including CYP3A4, that metabolize progestins may decrease the plasma concentrations of progestins, and may decrease
the effectiveness of progestin-only pills. Some drugs or herbal products that may decrease the effectiveness of progestin-only pills include:
• barbiturates
• bosentan
• carbamazepine
• felbamate
• griseofulvin
• oxcarbazepine
• phenytoin
• rifampin
• St. John’s wort
• topiramate
Significant changes (increase or decrease) in the plasma levels of the progestin have been noted in some cases of co-administration with HIV protease inhibitors or with
non-nucleoside reverse transcriptase inhibitors.
Consult the labeling of all concurrently used drugs to obtain further information about interactions with progestin-only pills or the potential for enzyme alterations.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Many studies have found no harmful effects on fetal development associated with long-term use of contraceptive doses of oral progestins. The few studies of
infant growth and development that have been conducted with progestin-only pills have not demonstrated significant adverse effects.
8.3 Nursing Mothers
In general, no adverse effects of progestin-only pills have been found on breastfeeding performance or on the health, growth, or development of the infant.
However, isolated post-marketing cases of decreased milk production have been reported. Small amounts of progestins pass into the breast milk of nursing
mothers taking progestin-only pills for long-term contraception, resulting in detectable steroid levels in infant plasma.
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Page 4
8.4 Pediatric Use
Safety and efficacy of progestin-only pills for long-term contraception have been established in women of reproductive age. Safety and efficacy are expected to
be the same for postpubertal adolescents less than 17 years and for users 17 years and older. Use of Plan B One-Step emergency contraception before menarche
is not indicated.
8.5 Geriatric Use
This product is not intended for use in postmenopausal women.
8.6 Race
No formal studies have evaluated the effect of race. However, clinical trials demonstrated a higher pregnancy rate in Chinese women with both Plan B and the
Yuzpe regimen (another form of emergency contraception). There was a non-statistically significant increased rate of pregnancy among Chinese women in the
Plan B One-Step trial. The reason for this apparent increase in the pregnancy rate with emergency contraceptives in Chinese women is unknown.
8.7 Hepatic Impairment
No formal studies were conducted to evaluate the effect of hepatic disease on the disposition of Plan B One-Step.
8.8 Renal Impairment
No formal studies were conducted to evaluate the effect of renal disease on the disposition of Plan B One-Step.
9 DRUG ABUSE AND DEPENDENCE
Levonorgestrel is not a controlled substance. There is no information about dependence associated with the use of Plan B One-Step.
10 OVERDOSAGE
There are no data on overdosage of Plan B One-Step, although the common adverse event of nausea and associated vomiting may be anticipated.
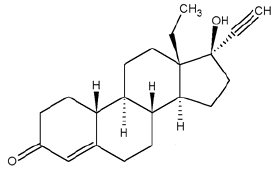
11 DESCRIPTION
The Plan B One-Step tablet contains 1.5 mg of a single active steroid ingredient, levonorgestrel [18,19-Dinorpregn-4-en-20-yn-3-one-13-ethyl-17-hydroxy-, (17
α)-(-)-], a totally synthetic progestogen. The inactive ingredients are colloidal silicon dioxide, corn starch, lactose monohydrate, magnesium stearate, potato
starch, and talc.
Levonorgestrel has a molecular weight of 312.45, and the following structural and molecular formulas:
C
21
H
28
O
2
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Emergency contraceptive pills are not effective if a woman is already pregnant. Plan B One-Step is believed to act as an emergency contraceptive principally by
preventing ovulation or fertilization (by altering tubal transport of sperm and/or ova). In addition, it may inhibit implantation (by altering the endometrium). It is
not effective once the process of implantation has begun.
12.3 Pharmacokinetics
Absorption
Following a single dose administration of Plan B One-Step in 30 women under fasting conditions, maximum plasma concentrations of levonorgestrel of 19.1
ng/mL were reached at 1.7 hours. See Table 2.
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Page 5
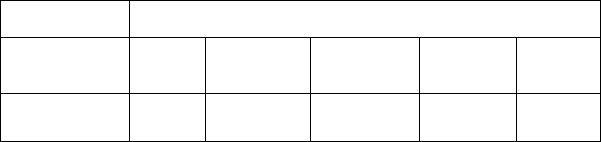
Table 2. Pharmacokinetic Parameter Values Following Single Dose Administration of Plan B One-Step
(levonorgestrel) tablet 1.5 mg to 30 Healthy Female Volunteers under Fasting Conditions
Mean (± SD)
C
max
(ng/mL)
AUC
t
(ng·hr/mL)*
AUC
inf
(ng·hr/mL)*
T
max
(hr)**
t
1/2
(hr)
Levonorgestrel
19.1
( 9.7)
294.8
( 208.8)
307.5
( 218.5)
1.7
(1.0-4.0)
27.5
( 5.6)
C
max
= maximum concentration
AUC
t
= area under the drug concentration curve from time 0 to time of last determinable concentration
AUC
inf
= area under the drug concentration curve from time 0 to infinity
T
max
= time to maximum concentration
t
1/2
= elimination half life
* N=29
** median (range)
Effect of Food: The effect of food on the rate and the extent of levonorgestrel absorption following single oral administration of Plan B One-Step has not been
evaluated.
Distribution
The apparent volume of distribution of levonorgestrel is reported to be approximately 1.8 L/kg. It is about 97.5 to 99% protein-bound, principally to sex hormone
binding globulin (SHBG) and, to a lesser extent, serum albumin.
Metabolism
Following absorption, levonorgestrel is conjugated at the 17β-OH position to form sulfate conjugates and, to a lesser extent, glucuronide conjugates in plasma.
Significant amounts of conjugated and unconjugated 3α, 5β-tetrahydrolevonorgestrel are also present in plasma, along with much smaller amounts of 3α, 5α-
tetrahydrolevonorgestrel and 16βhydroxylevonorgestrel. Levonorgestrel and its phase I metabolites are excreted primarily as glucuronide conjugates. Metabolic
clearance rates may differ among individuals by several-fold, and this may account in part for the wide variation observed in levonorgestrel concentrations among
users.
Excretion
About 45% of levonorgestrel and its metabolites are excreted in the urine and about 32% are excreted in feces, mostly as glucuronide conjugates.
Specific Populations
Pediatric
This product is not intended for use in the premenarcheal population, and pharmacokinetic data are not available for this population.
Geriatric
This product is not intended for use in postmenopausal women, and pharmacokinetic data are not available for this population.
Race
No formal studies have evaluated the effect of race. However, clinical trials demonstrated a higher pregnancy rate in Chinese women with both Plan B and the
Yuzpe regimen (another form of emergency contraception). There was a non-statistically significant increased rate of pregnancy among Chinese women in the
Plan B One-Step trial. The reason for this apparent increase in the pregnancy rate with emergency contraceptives in Chinese women is unknown [see USE IN
SPECIFIC POPULATIONS (8.6)].
Hepatic Impairment
No formal studies were conducted to evaluate the effect of hepatic disease on the disposition of Plan B One-Step.
Renal Impairment
No formal studies were conducted to evaluate the effect of renal disease on the disposition of Plan B One-Step.
Drug-Drug Interactions
No formal drug-drug interaction studies were conducted with Plan B One-Step [see DRUG INTERACTIONS (7)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity: There is no evidence of increased risk of cancer with short-term use of progestins. There was no increase in tumorgenicity following
administration of levonorgestrel to rats for 2 years at approximately 5 µg/day, to dogs for 7 years at up to 0.125 mg/kg/day, or to rhesus monkeys for
10 years at up to 250 µg/kg/day. In another 7 year dog study, administration of levonorgestrel at 0.5 mg/kg/day did increase the number of mammary
adenomas in treated dogs compared to controls. There were no malignancies.
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Page 6
Genotoxicity: Levonorgestrel was not found to be mutagenic or genotoxic in the Ames Assay, in vitro mammalian culture assays utilizing mouse
lymphoma cells and Chinese hamster ovary cells, and in an in vivo micronucleus assay in mice.
Fertility: There are no irreversible effects on fertility following cessation of exposures to levonorgestrel or progestins in general.
14 CLINICAL STUDIES
A double-blind, randomized, multicenter, multinational study evaluated and compared the efficacy and safety of three different regimens for emergency
contraception. Subjects were enrolled at 15 sites in 10 countries; the racial/ethnic characteristics of the study population overall were 54% Chinese, 34%
Caucasian, and 12% Black or Asian (other than Chinese). 2,381 healthy women with a mean age of 27 years, who needed emergency contraception within 72
hours of unprotected intercourse were involved and randomly allocated into one of the two levonorgestrel groups. A single dose of 1.5 mg of levonorgestrel (Plan
B One-Step) was administered to women allocated into group 1. Two doses of 0.75 mg levonorgestrel 12 hours apart (Plan B) were administered to women in
group 2. In the Plan B One-Step group, 16 pregnancies occurred in 1,198 women and in the Plan B group, 20 pregnancies occurred in 1,183 women. The number
of pregnancies expected in each group was calculated based on the timing of intercourse with regard to each woman’s menstrual cycle. Among women receiving
Plan B One-Step, 84% of expected pregnancies were prevented and among those women taking Plan B, 79% of expected pregnancies were prevented. The
expected pregnancy rate of 8% (with no contraceptive use) was reduced to approximately 1% with Plan B One-Step.
Emergency contraceptives are not as effective as routine contraception since their failure rate, while low based on a single use, would accumulate over time with
repeated use [see INDICATIONS AND USAGE (1)].
In the clinical study, bleeding disturbances were the most common adverse event reported after taking the levonorgestrel-containing regimens. More than half of
the women had menses within two days of the expected time; however, 31% of women experienced change in their bleeding pattern during the study period;
4.5% of women had menses more than 7 days after the expected time.
16 HOW SUPPLIED/STORAGE AND HANDLING
The Plan B One-Step (levonorgestrel) tablet 1.5 mg is available in a PVC/aluminum foil blister package. The tablet is almost white, round, and marked G00 on
one side.
NDC 51285-088-88 (1 tablet unit of use package)
Store Plan B One-Step at 20º to 25ºC (68º to 77ºF) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
17.1 Information for Patients
• Take Plan B One-Step as soon as possible and not more than 72 hours after unprotected intercourse or a known or suspected contraceptive failure.
• If you vomit within two hours of taking the tablet, immediately contact your healthcare provider to discuss whether to take another tablet.
• Seek medical attention if you experience severe lower abdominal pain 3 to 5 weeks after taking Plan B One-Step, in order to be evaluated for an ectopic
pregnancy.
• After taking Plan B One-Step, consider the possibility of pregnancy if your period is delayed more than one week beyond the date you expected your period.
• Do not use Plan B One-Step as routine contraception.
• Plan B One-Step is not effective in terminating an existing pregnancy.
• Plan B One-Step does not protect against HIV-infection (AIDS) and other sexually transmitted diseases/infections.
• For women younger than age 17 years, Plan B One-Step is available only by prescription.
Mfg. by Gedeon Richter, Ltd., Budapest, Hungary
for Duramed Pharmaceuticals, Inc.
Subsidiary of Barr Pharmaceuticals, Inc.
Pomona, New York 10970
Phone: 1-800-330-1271
Website: www.PlanBOneStep.com
Revised: July 2009
11001459
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

(
levonorgestrel) tablet 1 5 mg
For additional information intended for healthcare
professionals, please see enclosed Product
Information for Plan B
®
One-Step.
Plan B
®
One-Step
Emergency Contraceptive.
Because the unexpected happens.
Important Information About Plan B
®
One-Step,
Birth Control & Sexually Transmitted Diseases
, .
®
Emergency
Contraceptive
From the makers of Plan B
®
, Duramed Pharmaceuticals, Inc., a subsidiary of Barr Pharmaceuticals, Inc.
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

What is Plan B
®
One-Step?
Plan B
®
One-Step is emergency contraception that helps prevent
pregnancy after birth control failure or unprotected sex. It is a backup
method of preventing pregnancy and is not to be used routinely.
Plan B
®
One-Step can reduce your chance of pregnancy after
unprotected sex (if your regular birth control was used incorrectly or
fails, or if you have had sex without birth control). For example, if you
were using a condom and it broke or slipped, if you did not use your
regular birth control as you should have, or if you did not use any
birth control, Plan B
®
One-Step may work for you.
2
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

(
levonorgestrel) tablet 1 5 mg
3
, .
®
What Plan B
®
One-Step is not.
Plan B
®
One-Step will not work if you are already pregnant and will
not affect an existing pregnancy. Plan B
®
One-Step should not be
used as regular birth control. It is important to have another reliable
source of birth control that is right for you. Plan B
®
One-Step will not
protect you from HIV infection (the virus that causes AIDS) and other
sexually transmitted diseases.
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

When is the appropriate time to use Plan B
®
One-Step?
You can use Plan B
®
One-Step after you have had unprotected sex in
the last 72 hours (3 days), and you do not want to become pregnant.
Plan B
®
One-Step can be used as a backup or emergency method to
regular birth control if, for example,
• Your regular birth control method was used incorrectly or failed
(your partner’s condom broke or slipped)
• You made a mistake with your regular method
• You did not use any birth control method
4
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

(
levonorgestrel) tablet 1 5 mg, .
®
5
When is it not appropriate to use Plan B
®
One-Step?
• Plan B
®
One-Step should not be used as a regular birth control
method. It does not work as well as most other forms of birth
control when they are used consistently and correctly. Plan B
®
One-Step is a backup or emergency method of contraception.
• Plan B
®
One-Step should not be used if you are already pregnant
because it will not work.
• Plan B
®
One-Step should not be used if you are
allergic to levonorgestrel or any other ingredients
in Plan B
®
One-Step.
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

• Plan B
®
One-Step does not protect against HIV (the virus that causes
AIDS) or other sexually transmitted diseases (STDs). The best ways to
protect yourself against getting HIV or other STDs are to use a latex
condom correctly with every sexual act or not to have sex at all.
6
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

(
levonorgestrel) tablet 1 5 mg
7
, .
®
How does Plan B
®
One-Step work?
Plan B
®
One-Step is one pill with levonorgestrel, a hormone that
has been used in many birth control pills for over 35 years. Plan B
®
One-Step contains a higher dose of levonorgestrel than birth control
pills, but works in a similar way to prevent pregnancy. It works mainly
by stopping the release of an egg from the ovary. It is possible that
Plan B
®
One-Step may also work by preventing fertilization of an egg
(the uniting of sperm with the egg) or by preventing attachment
(implantation) to the uterus (womb).
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

How can I get the best results from Plan B
®
One-Step?
You have only a few days to try to prevent pregnancy after
unprotected sex. The sooner you take Plan B
®
One-Step, the better
it works. Plan B
®
One-Step should be taken as soon as possible
within 72 hours (3 days) after unprotected sex.
How effective is Plan B
®
One-Step?
The sooner you take Plan B
®
One-Step, the better it will work. Take Plan B
®
One-Step as soon as possible after unprotected sex. If it is taken as soon as
possible within 72 hours (3 days) after unprotected sex, it will significantly
decrease the chance that you will get pregnant. Seven out of every 8 women
who would have gotten pregnant will not become pregnant.
8
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

(
levonorgestrel) tablet 1 5 mg
9
, .
®
How will I know if Plan B
®
One-Step worked?
Most women will have their next menstrual period at the expected
time or within a week of the expected time. If your menstrual period
is delayed beyond 1 week, you may be pregnant. You should get a
pregnancy test and follow up with your healthcare professional.
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

What if I am already pregnant and use
Plan B
®
One-Step?
There is no medical evidence that Plan B
®
One-Step would harm a
developing baby. If you take Plan B
®
One-Step (accidentally) after you
are already pregnant or it does not work and you become pregnant,
it is not likely to cause any harm to you or your pregnancy. The
pregnancy will continue. Plan B
®
One-Step will not work if you are
already pregnant.
10
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

(
levonorgestrel) tablet 1 5 mg
11
, .
®
What should I do if my menstrual period is
delayed beyond 1 week and I have severe
lower stomach (abdominal) pain?
If you have severe lower stomach (abdominal) pain about 3 to 5 weeks
after taking Plan B
®
One-Step, you may have a pregnancy outside the
uterus, which is called a tubal pregnancy. A tubal pregnancy requires
immediate medical treatment, so you should see a healthcare
professional right away.
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Can I use Plan B
®
One-Step for regular birth control?
No. Plan B
®
One-Step should not be used for regular birth control. It
is an emergency or backup method to be used if your regular birth
control fails or is used incorrectly or if you have sex without birth
control. You should protect yourself against STDs and pregnancy
every time you have sex. If you have unprotected sex again after
taking Plan B
®
One-Step, it will not help protect you from getting
pregnant.
12
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

(
levonorgestrel) tablet 1 5 mg
13
, .
®
How often can I use Plan B
®
One-Step?
Plan B
®
One-Step is meant for emergency protection only, and is not
designed to be used frequently. If you find that you need to use
emergency contraception often, talk to your healthcare professional
and learn about methods of birth control and STD prevention that
are right for you.
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Will I experience any side effects from Plan B
®
One-Step?
When used as directed, Plan B
®
One-Step is safe for women. Some
women will have mild, temporary side effects, such as menstrual
changes, nausea, lower stomach (abdominal) pain, tiredness,
headache, dizziness, breast pain and vomiting. These are similar to
the side effects that some women have when taking regular birth
control pills. Some women taking Plan B
®
One-Step will have
menstrual changes such as spotting or bleeding before their next
period. Some women may have a heavier or lighter next period, or a
period that is early or late. If your period is more than a week late,
you should get a pregnancy test.
14
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

(
levonorgestrel) tablet 1 5 mg
15
, .
®
15
What warnings should I know about when using
Plan B
®
One-Step?
Plan B
®
One-Step does not protect against the AIDS virus (HIV)
or other sexually transmitted diseases (STDs).
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Do not use:
• If you are already pregnant (because it will not work)
• If you are allergic to levonogestrel or any of the ingredients in Plan B
®
One-Step
• For regular birth control
When using this product, you may have:
• Menstrual changes • Headache
• Nausea • Dizziness
•
Lower stomach (abdominal) pain
• Breast pain
• Tiredness • Vomiting
1616
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control
Center right away at 1-800-222-1222.
What are the directions for using Plan B
®
One-Step?
Women 17 years of age and older:
• Take Plan B
®
One-Step as soon as possible within 72 hours (3 days)
after unprotected sex.
• If you vomit within 2 hours of taking the medication, call a
healthcare professional to find out if you should repeat the dose.
Prescription only for women younger than age 17. If you are younger
than age 17, see a healthcare professional.
17
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

What should I do if I have questions about
Plan B
®
One-Step?
If you have questions or need more information about this product,
call our toll-free number, 1-800-330-1271, visit our website at
www.PlanBOneStep.com, or ask a healthcare professional.
Other information
Tablet is enclosed in a blister seal. Do not use if the blister seal
is broken.
Store at room temperature 20–25°C (68–77°F).
You may report side effects to FDA at 1-800-FDA-1088.
1818
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

(
levonorgestrel) tablet 1 5 mg
19
, .
®
19
Active ingredient: levonorgestrel 1.5 mg
Inactive ingredients: colloidal silicon dioxide, potato starch,
magnesium stearate, talc, corn starch, lactose monohydrate
Protect yourself in more ways than one!
If you are sexually active, but you are not ready for a pregnancy, it is
important to use regular pregnancy protection. There are many types
of birth control. Whichever type you choose, it is important to use your
regular birth control method as directed.
This ensures that you have effective
protection against pregnancy
every time you have sex.
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

But things do not always go as planned. For example, if you were
using a condom and it broke or slipped, or if you did not use your
regular birth control as you should have, or if you did not use any
birth control, Plan B
®
One-Step may work for you. Plan B
®
One-Step is
an emergency contraceptive that helps prevent pregnancy after
unprotected sex or when your birth control fails or is not used
correctly.
Remember, Plan B
®
One-Step is only for emergency pregnancy
prevention. There are many other products that work for regular birth
control that are available by prescription or over-the-counter.
2020
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

(
levonorgestrel) tablet 1 5 mg
21
, .
®
21
There is also another form of protection to think about when you have
sex: protection against sexually transmitted diseases (STDs). Some
common STDs are HIV/AIDS, chlamydia, genital herpes, gonorrhea,
hepatitis, human papilloma virus (HPV), genital warts, syphilis, and
trichomonas. Some of these STDs can be very serious and can lead to
infertility (inability to have a baby), problems during pregnancy,
chronic illness, and even death.
All sexually active women are at risk of catching STDs because they
may not know that their partner has an STD (the partner
himself may not know). If your partner uses a
latex condom correctly each and every time
you have sex with him, this will help reduce,
but not eliminate, the chance that you will
catch an STD.
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

No other birth control methods will effectively protect you from STDs.
The female condom may give you some STD protection, but it is not
as effective as a male latex condom.
For more information on STDs, call the Centers for Disease Control
and Prevention (CDC) AIDS/STD Hotline. The CDC phone numbers
are 1-800-342-AIDS (2437) for English, 1-800-344-7432 for Spanish, or
1-800-243-7889 for hearing impaired, TDD.
Be sure to protect yourself against pregnancy and STDs by using
some form of birth control plus a latex condom. Of course, not having
sex is the most effective way to prevent pregnancy and stay free of
STDs.
2222
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

(
levonorgestrel) tablet 1 5 mg
23
, .
®
23
Plan B
®
One-Step is used to prevent pregnancy after unprotected sex.
Plan B
®
One-Step should not be used for regular birth control, if
you are already pregnant (because it will not work), or if you are
allergic to levonorgestrel or any of the ingredients in Plan B
®
One-Step.
The sooner you take Plan B
®
One-Step, the better it will work.
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Plan B
®
One-Step does not protect against the AIDS virus (HIV)
or other sexually transmitted diseases (STDs)
Common side effects associated with the use of Plan B
®
One-Step
include
menstrual changes, nausea, lower stomach (abdominal) pain,
tiredness, headache, dizziness, breast pain and vomiting
.
24
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

(
levonorgestrel) tablet 1 5 mg
1-800-330-1271
Mfg. by Gedeon Richter, Ltd., Budapest, Hungary
for Duramed Pharmaceuticals, Inc.
Subsidiary of Barr Pharmaceuticals, Inc.
Pomona, New York 10970
©2009 Duramed Pharmaceuticals, Inc. 12001119
®
www.PlanBOneStep.com
, .
®
July 2009 Printed in USA
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
t
t
after unprotected sex. The sooner you tak
the better Plan B® One-Step will work.
NDC 51285-088-88
One
One
e
Tablet
levonorgestrel) tablet, 1.5 mg
Emergency Contraceptive
®
levonorgestrel) tablet, 1.5 mg
Emergency Contraceptive
Le
yo
Emergency
Contraceptive
Emergency
Contraceptivet
i
Le
yo
Emergency
Contraceptive
t
i
Emergency
Contraceptive
i
•
Not for regular birth control.
Plan B® One-Step should be used only in emergencies.
•
Does not protect against HIV/AIDS or other STDs.
m
er
g
encies.
e
r STDs
.
1
Tablet Levonorgestrel 1.5mg
©2005 Duramed Pharmaceuticals, Inc.
•
Take as soon as possible within 72 hours (3 days)
e it,
Levonorgestrel Tablet 1.5mg
p
(
after unprotected sex. The sooner you take
t
he better Plan B® One-Step w
i
ll work.
On
e
e
ne
e
e
e
One
Tablet
Tablet
Dose
Dose
Reduces the chance of pregnancy after
unprotected sex (if a regular birth control
method fails or after sex without birth control)
Not for regular birth control.
•
Take as soon as possible
within 72 hours (3 days)
after unprotected sex.
The sooner you take it, the
better Plan B® One-Step
will work.
Ta
Ta
bl
et
1
Levonorgestrel 1.5mg
(
vonorgestr
ND
C
5
1285
(
(
l
l
evonorges
t
re
l
l
)
)
t
t
a
b
b
l
l
et,
1
1
.
5
5
m
g
Emer
g
ency Contracept
i
ve
®
NEW!
5-
088
-88
®
E
W
!
Now only
ONE
dose
Levonorgestrel Tablet 1.5mg
(
levonorgestrel) tablet, 1.5 mg
Emergency Contraceptive
(
(
(
l
l
evonorgestre
l
l
)
)
t
a
b
b
l
l
et,
1
1
.
5
5
m
g
Emer
g
ency Contraceptive
®
vonorgestrel Tablet 1.5mg
Rx only for women
unger than age 17
Levon
o
R
x o
n
yo
un
g
E
mergency
Contracept
ive
(
levonorgestrel) tablet, 1.5 mg
®
Emergenc
y
Contrace
p
ve
(
levonorgestrel) tablet, 1.5 mg
®
el Tablet 1.5mg
Rx only for women
unger than age 17
L
evon
o
R
x o
n
youn
g
Emer
g
enc
y
Contrace
p
ve
(
levonorgestrel) tablet, 1.5 mg
®
E
mergency
C
ontracept v
e
(
levonorgestrel) tablet, 1.5 mg
®
Rx only for women
younger than age 17
Mfg. by Gedeon Richter, Ltd., Budapest, Hungary
for Duramed Pharmaceuticals, Inc.
Subsidiary of Barr Pharmaceuticals, Inc.
Pomona, New York 10970
51285 08888
83
N
15001332 Iss. 7/09
Drug Facts
Active ingredient Purpose
Levonorgestrel 1.5mg........................Emergency contraceptive
Use
reduces chance of pregnancy after unprotected sex
(if a contraceptive failed or if you did not use birth control)
Warnings
Allergy alert:
Do not use if you have ever had an allergic
reaction to levonorgestrel
Sexually transmitted diseases (STDs) alert: This product
does not protect against HIV/AIDS or other STDs
Do not use
■
if you are already pregnant (because it will not work)
■
for regular birth control
Drug Facts (continued)
Directions
■
women 17 years of age or older:
Keep out of reach of children. In case of overdose, get medical
help or contact a Poison Control center right away.
Other information
■
before using this product read the enclosed consumer
information leaflet for complete directions and information
■
this product is not recommended for regular birth control. It
does not work as well as most other birth control methods
used correctly.
■
this product works mainly by preventing ovulation (egg
release). It may also prevent fertilization of a released egg
(joining of sperm and egg) or attachment of a fertilized egg
to the uterus (implantation).
■
when used correctly every time you have sex, latex condoms
greatly reduce, but do not eliminate, the risk of pregnancy and
the risk of catching or spreading HIV, the virus that causes AIDS.
See condom labeling for additional STD information
.
■
tablet is enclosed in a blister seal
.
Do not use if the
blister
seal is broken.
■
store at 20-25°C (68-77°F)
■
take tablet as soon as possible within 72 hours (3 days)
after unprotected sex. The sooner you take it the
better it will work.
■
if you vomit within 2 hours of taking the medication, call a
healthcare professional to find out if you should repeat the dose
Inactive ingredients
colloidal silicon dioxide, corn starch, lactose
monohydrate, magnesium stearate, potato starch, talc
Questions or comments?
For more information or to speak to a healthcare professional,
call 1-800-330-1271, 24 hours a day/7 days a week.
Visit our Web site at
www.PlanBOneStep.com
■
prescription only for women younger than age 17. If you
are younger than age 17, see a healthcare professional.
When using this product you may have
■
menstrual changes
■
nausea
■
lower stomach (abdominal) pain
■
tiredness
■
headache
■
dizziness
■
breast pain
■
vomiting
DRUG FACTS TEXT DEFINED
• DRUG FACTS Title
• DRUG FACTS CONTINUED
• HEADINGS
• SUBHEADS/BODY TEXT
• LEADING
• # OF CHARACTERS PER INCH
• BULLETS
• SPACE AFTER BULLETED SECTION
• BARLINES, HAIRLINES
• SPACE BETWEEN HAIRLINES AND BOX END
TYPE SIZE
9 pt
8 pt
8 pt
6 pt
7 pt
<39
5 pt
2 ems
1 pt, .5 pt
2 spaces
TYPE FONT
Helvetica Bold Condensed Oblique
Helvetica Bold Cond. Oblique/Helvetica Condensed
Helvetica Bold Condensed Oblique
Helvetica Bold Condensed/Helvetica Condensed
Zapf Dingbats
PMS 2726 PMS 375 PMS 7486 PMS 245 BLACK PMS 327 PMS 288
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

gnancy a
after unprotected sex. The sooner you tak
the better Plan B® One-Step will work.
yo
Levonorgestrel Tablet 1.5mg
(
levonorgestrel) tablet, 1.5 mg
Emergency Contraceptive
(
)
1
(
levonorgestrel) tablet, 1.5 mg
Emergency Contraceptive
(
l
.
i
Le
yo
Emergency
Contraceptive
E
enc
i
ve
Emergency
Contraceptive
i
ve
Le
yo
Emergency
Contraceptive
enc
t
i
ve
Emergency
Contraceptive
E
e
NDC 51285-
088
-19
(
levonorgestrel) tablet, 1.5 mg
Emergency Contraceptive
Ta
One
One
One
One
•
Take as soon as possible within 72 hours (3 days)
e it,
Rx only for women
unger than age 17
•
Not for regular birth control.
Plan B® One-Step should be used only in emergencies.
•
Does not protect against HIV/AIDS or other STDs.
me
r
g
encies
.
er
STDs.
1
Tablet Levonorgestrel 1.5mg
©2005 Duramed Pharmaceuticals, Inc.
p (
after un
p
rotected sex. The sooner
y
ou ta
k
the better Plan B® One-Ste
p
will work.
levonorgestrel tablet, .5 m
g
Emer
g
ency Contraceptive
®
Levonorgestrel Tablet 1.5mg
evonorgestrel) tablet,
1
5
m
g
Emergency Contracept ve
®
vonorgestrel Tablet 1.5mg
Rx only for women
unger than age 17
Levon
o
R
x o
n
yo
un
g
merg
y
Contracept
(
levonorgestrel) tablet, 1.5 mg
®
E
mergenc
y
Contracept
(
levonorgestrel) tablet, 1.5 mg
®
vonorgestrel Tablet 1.5mg
Rx only for women
unger than age 17
L
evon
o
R
x o
n
yo
un
g
Emer
g
y
Contrace
p
(
levonorgestrel) tablet, 1.5 mg
®
mergency
C
ontracept
iv
(
levonorgestrel) tablet, 1.5 mg
®
Reduces the chance of pregnancy after
unprotected sex (if a regular birth control
method fails or after sex without birth control)
Not for regular birth control.
Levonor
g
estrel Tablet 1.5m
g
N
D
C
5
128
Reduces the chance of pre fter
regnancy afte
Emergency Contraceptive
traceptive
NEW!
8
5-
088
-
19
E
W
!
Now only ONE dose
®
FOR CLINIC USE ONLY. NOT FOR RESALE.
blet Levonorgestrel 1.5mgTablet
L
1
yo
unge
than age 17
er than age
yo
unge
y
g
Tablet
Tablet
Dose
Dose
•
Take as soon as possible
within 72 hours (3 days)
after unprotected sex.
The sooner you take it, the
better Plan B® One-Step
will work.
Mfg. by Gedeon Richter, Ltd., Budapest, Hungary
for Duramed Pharmaceuticals, Inc.
Subsidiary of Barr Pharmaceuticals, Inc.
Pomona, New York 10970
51285 08819
23
N
15001331 Iss. 7/09
Drug Facts
Active ingredient Purpose
Levonorgestrel 1.5mg........................Emergency contraceptive
Use
reduces chance of pregnancy after unprotected sex
(if a contraceptive failed or if you did not use birth control)
Warnings
Allergy alert:
Do not use if you have ever had an allergic
reaction to levonorgestrel
Sexually transmitted diseases (STDs) alert: This product
does not protect against HIV/AIDS or other STDs
Do not use
■
if you are already pregnant (because it will not work)
■
for regular birth control
Drug Facts (continued)
Directions
■
women 17 years of age or older:
Keep out of reach of children. In case of overdose, get medical
help or contact a Poison Control center right away.
Other information
■
before using this product read the enclosed consumer
information leaflet for complete directions and information
■
this product is not recommended for regular birth control. It
does not work as well as most other birth control methods
used correctly.
■
this product works mainly by preventing ovulation (egg
release). It may also prevent fertilization of a released egg
(joining of sperm and egg) or attachment of a fertilized egg
to the uterus (implantation).
■
when used correctly every time you have sex, latex condoms
greatly reduce, but do not eliminate, the risk of pregnancy and
the risk of catching or spreading HIV, the virus that causes AIDS.
See condom labeling for additional STD information
.
■
tablet is enclosed in a blister seal
.
Do not use if the
blister
seal is broken.
■
store at 20-25
°
C (68-77
°
F)
■
take tablet as soon as possible within 72 hours (3 days)
after unprotected sex. The sooner you take it the
better it will work.
■
if you vomit within 2 hours of taking the medication, call a
healthcare professional to find out if you should repeat the dose
Inactive ingredients
colloidal silicon dioxide, corn starch, lactose
monohydrate, magnesium stearate, potato starch, talc
Questions or comments?
For more information or to speak to a healthcare professional,
call
1-800-330-1271,
24 hours a day/7 days a week.
Visit our Web site at
www.PlanBOneStep.com
■
prescription only for women younger than age 17. If you
are younger than age 17, see a healthcare professional.
When using this product you may have
■
menstrual changes
■
nausea
■
lower stomach (abdominal) pain
■
tiredness
■
headache
■
dizziness
■
breast pain
■
vomiting
DRUG FACTS TEXT DEFINED
• DRUG FACTS Title
• DRUG FACTS CONTINUED
• HEADINGS
• SUBHEADS/BODY TEXT
• LEADING
• # OF CHARACTERS PER INCH
• BULLETS
• SPACE AFTER BULLETED SECTION
• BARLINES, HAIRLINES
• SPACE BETWEEN HAIRLINES AND BOX END
TYPE SIZE
9 pt
8 pt
8 pt
6 pt
7 pt
<39
5 pt
2 ems
1 pt, .5 pt
2 spaces
TYPE FONT
Helvetica Bold Condensed Oblique
Helvetica Bold Cond. Oblique/Helvetica Condensed
Helvetica Bold Condensed Oblique
Helvetica Bold Condensed/Helvetica Condensed
Zapf Dingbats
PMS 375 BLACK PMS 327 PMS 288
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
